The Inert Nature Of Noble Gases: How A Full Octet Shapes Their Behavior
The Inert Nature of Noble Gases: How a Full Octet Shapes Their Behavior
Related Articles: The Inert Nature of Noble Gases: How a Full Octet Shapes Their Behavior
Introduction
With great pleasure, we will explore the intriguing topic related to The Inert Nature of Noble Gases: How a Full Octet Shapes Their Behavior. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Inert Nature of Noble Gases: How a Full Octet Shapes Their Behavior

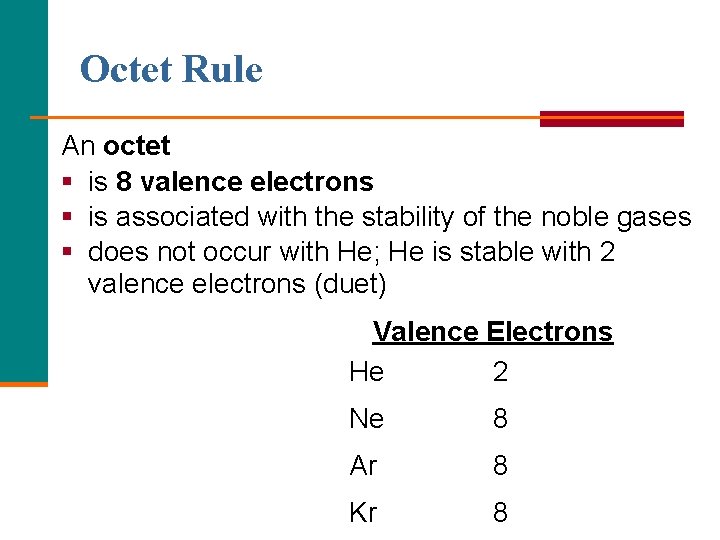
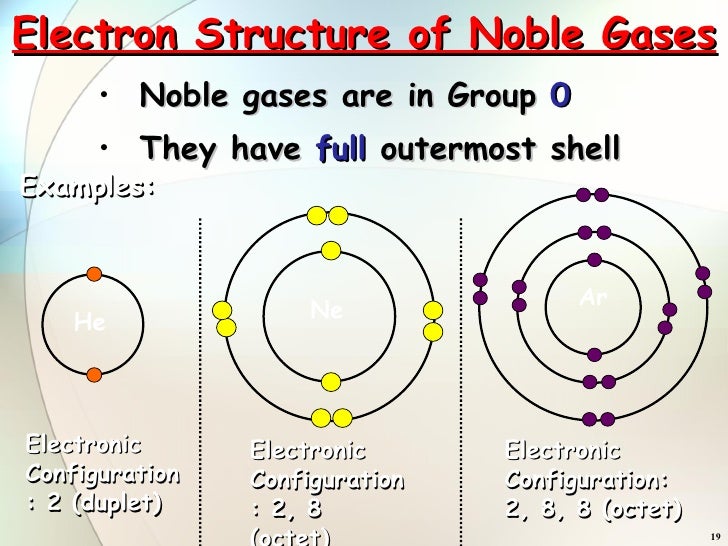
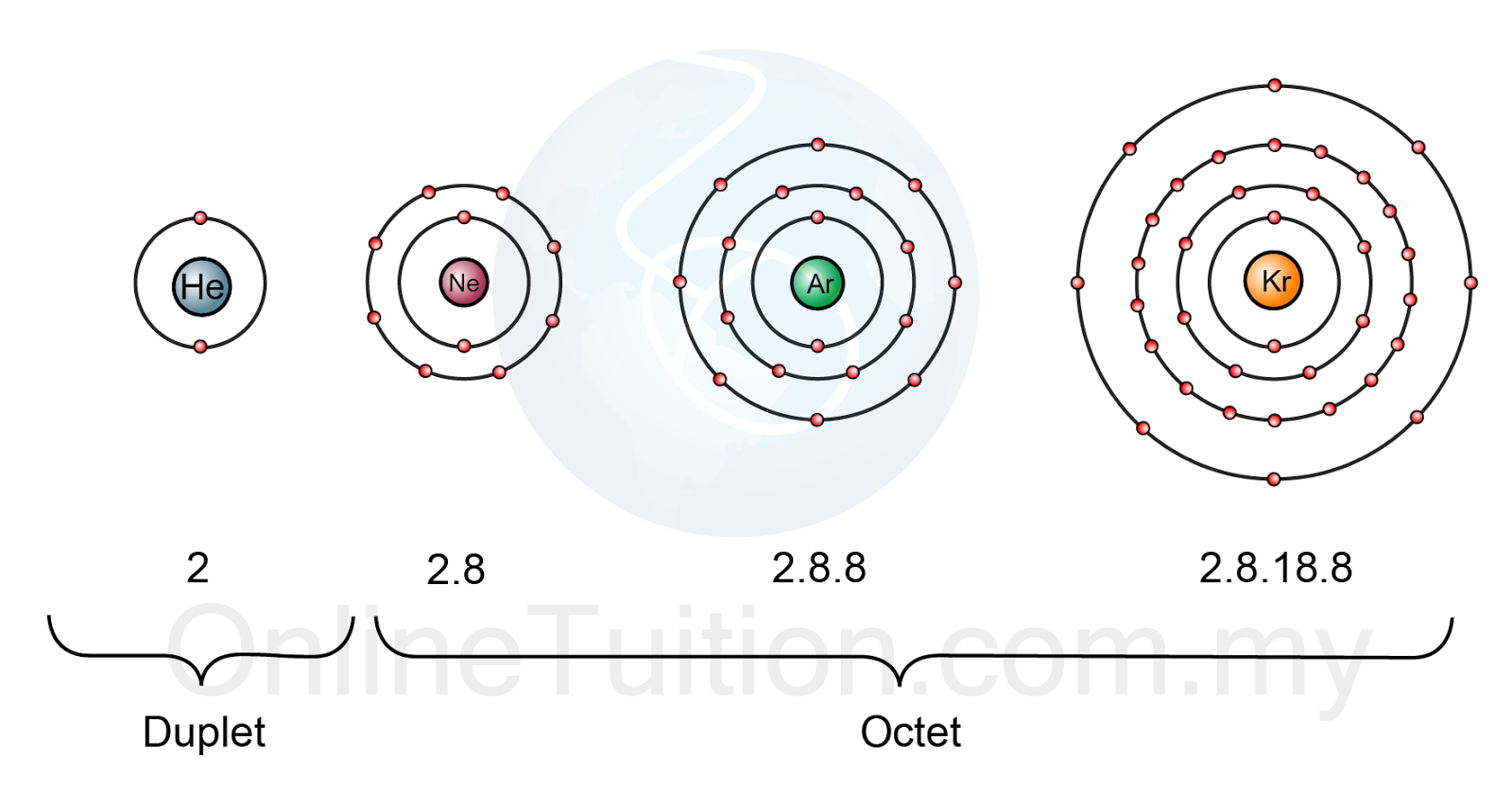
The noble gases, also known as inert gases, occupy Group 18 of the periodic table. These elements, including helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn), are characterized by their exceptional stability and lack of reactivity. This unique behavior stems from their electron configuration, specifically the presence of a full octet in their outermost electron shell.
Understanding the Octet Rule
The octet rule, a fundamental principle in chemistry, states that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight electrons in their outermost shell, mimicking the electron arrangement of the noble gases. This stable configuration, with a full octet, minimizes the atom’s energy, making it less likely to participate in chemical reactions.
The Impact of a Full Octet on Noble Gas Properties
The presence of a full octet has profound implications for the properties of noble gases, influencing their:
- Low Reactivity: Noble gases are renowned for their inert nature. Their full octet renders them exceptionally stable, with minimal tendency to form chemical bonds with other elements. This inertness makes them ideal for applications where chemical reactions are undesirable, such as in lighting and as protective atmospheres.
- High Ionization Energy: Due to their stable electron configuration, noble gases require significant energy to remove an electron. This high ionization energy further contributes to their unreactive nature.
- Weak Interatomic Forces: The lack of readily available electrons for bonding results in weak interatomic forces between noble gas atoms. This translates to low boiling points and melting points, making them gases at room temperature.
- Colorless, Odorless, and Tasteless: Noble gases are typically colorless, odorless, and tasteless under standard conditions. Their lack of reactivity contributes to their lack of strong interactions with light, smell receptors, or taste buds.
Exploring the Trends in Noble Gas Properties
While noble gases are known for their inert nature, subtle trends emerge within the group as we move down the periodic table. These trends are primarily driven by:
- Increasing Atomic Radius: As we descend the group, the atomic radius increases due to the addition of electron shells. This larger size increases the distance between the nucleus and the outermost electrons, leading to a decrease in ionization energy.
- Decreasing Ionization Energy: The larger atomic radius and the shielding effect of inner electrons make it easier to remove an electron from heavier noble gases. This decrease in ionization energy is reflected in the increasing reactivity of heavier noble gases, as they can participate in some chemical reactions.
- Increased Polarizability: The larger atomic radius and increased number of electrons in heavier noble gases make their electron clouds more deformable. This increased polarizability allows heavier noble gases to form weak interactions with other molecules, leading to the formation of some compounds.
The Future of Noble Gases
The unique properties of noble gases have led to their increasing importance in various fields. As we move towards a more sustainable and technologically advanced future, their applications are likely to expand further:
- Lighting: Noble gases, particularly neon, argon, and krypton, are widely used in lighting applications. They produce vibrant colors when excited by an electric current, creating neon signs and other decorative lighting fixtures.
- Protective Atmospheres: The inert nature of noble gases makes them ideal for creating protective atmospheres in various applications. They are used to prevent oxidation and corrosion in welding, semiconductor manufacturing, and food packaging.
- Medical Applications: Noble gases, particularly helium, are used in medical imaging and respiratory therapy. Their unique properties enable them to enhance diagnostic procedures and provide therapeutic benefits.
- Emerging Technologies: Research continues to explore novel applications of noble gases in fields like lasers, nuclear power, and advanced materials. Their versatility and unique properties hold promise for future technological advancements.
Related Searches
- Noble Gas Applications: This search explores the diverse applications of noble gases in various industries, highlighting their importance in modern technology.
- Noble Gas Chemistry: This search delves into the chemical behavior of noble gases, discussing their reactivity, compound formation, and the factors influencing their bonding.
- Noble Gas Properties: This search provides a comprehensive overview of the physical and chemical properties of noble gases, explaining their unique characteristics and the trends observed within the group.
- Noble Gas History: This search explores the historical discovery and characterization of noble gases, highlighting the scientific breakthroughs that led to their understanding.
- Noble Gas Abundance: This search examines the relative abundance of noble gases in the Earth’s atmosphere and crust, discussing their sources and distribution.
- Noble Gas Isotopes: This search focuses on the different isotopes of noble gases, their properties, and their applications in fields like dating and environmental monitoring.
- Noble Gas Spectroscopy: This search explores the use of spectroscopic techniques to study the energy levels and electronic transitions of noble gases, providing insights into their atomic structure.
- Noble Gas Environmental Impact: This search examines the potential environmental impacts of noble gas emissions, particularly from industrial processes and nuclear power plants.
FAQs
Q: Why are noble gases called inert gases?
A: Noble gases are called inert gases because they are exceptionally unreactive due to their stable electron configuration with a full octet in their outermost shell. This stable configuration makes them reluctant to participate in chemical reactions.
Q: Do noble gases ever form compounds?
A: While noble gases are generally considered inert, heavier noble gases like xenon and krypton have been observed to form compounds under specific conditions. These compounds are typically formed with highly electronegative elements like fluorine and oxygen.
Q: What are some practical applications of noble gases?
A: Noble gases have a wide range of applications, including:
- Lighting: Neon signs, argon-filled fluorescent lights, and krypton-filled high-intensity lamps.
- Protective Atmospheres: Inert gas atmospheres are used in welding, semiconductor manufacturing, and food packaging to prevent oxidation and corrosion.
- Medical Applications: Helium is used in respiratory therapy and medical imaging.
Q: How does the full octet affect the boiling point of noble gases?
A: The full octet leads to weak interatomic forces between noble gas atoms. These weak forces result in low boiling points, making them gases at room temperature. As we move down the group, the boiling point increases due to the larger size and increased polarizability of heavier noble gases.
Q: What are the future prospects of noble gas research?
A: Research continues to explore novel applications of noble gases in various fields, including:
- Lasers: Noble gas lasers are used in various applications, including medical surgery, material processing, and scientific research.
- Nuclear Power: Noble gases are used in nuclear power plants as cooling agents and in the detection of radioactive materials.
- Advanced Materials: Noble gases are being investigated for their potential use in developing novel materials with unique properties.
Tips
- Understanding the Octet Rule: Begin by grasping the fundamental principle of the octet rule, which explains the stability of noble gases and their inert behavior.
- Focus on Trends: Explore the trends in properties within the noble gas group, such as increasing atomic radius and decreasing ionization energy, to understand the subtle variations in their reactivity.
- Applications and Future Prospects: Investigate the diverse applications of noble gases in various fields, highlighting their current and potential future contributions to technology and society.
Conclusion
The full octet of electrons in the outermost shell of noble gases is the cornerstone of their unique inert nature. This stable configuration renders them exceptionally unreactive, leading to their distinctive properties, including low boiling points, high ionization energies, and weak interatomic forces. While traditionally considered inert, heavier noble gases exhibit some reactivity under specific conditions, expanding their potential applications in chemistry and technology. The remarkable properties of noble gases, driven by their full octet, continue to inspire scientific exploration and unlock new possibilities for their use in diverse fields, from lighting and medicine to advanced materials and energy technologies.





Closure
Thus, we hope this article has provided valuable insights into The Inert Nature of Noble Gases: How a Full Octet Shapes Their Behavior. We thank you for taking the time to read this article. See you in our next article!