Understanding Periodic Trends: A Comprehensive Guide
Understanding Periodic Trends: A Comprehensive Guide
Related Articles: Understanding Periodic Trends: A Comprehensive Guide
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Understanding Periodic Trends: A Comprehensive Guide. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding Periodic Trends: A Comprehensive Guide
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
The periodic table is a fundamental tool in chemistry, organizing elements based on their atomic structure and recurring properties. Understanding these recurring properties, known as periodic trends, is crucial for predicting chemical behavior and understanding how elements interact.
Periodic Trends Gizmo is a valuable resource for students and educators, providing an interactive platform to explore these trends visually and practically. While a specific answer key for a 2025 version of the Gizmo might not be readily available, the underlying principles and concepts remain constant. This article aims to provide a comprehensive understanding of periodic trends and how to utilize resources like the Gizmo effectively.
Exploring Periodic Trends
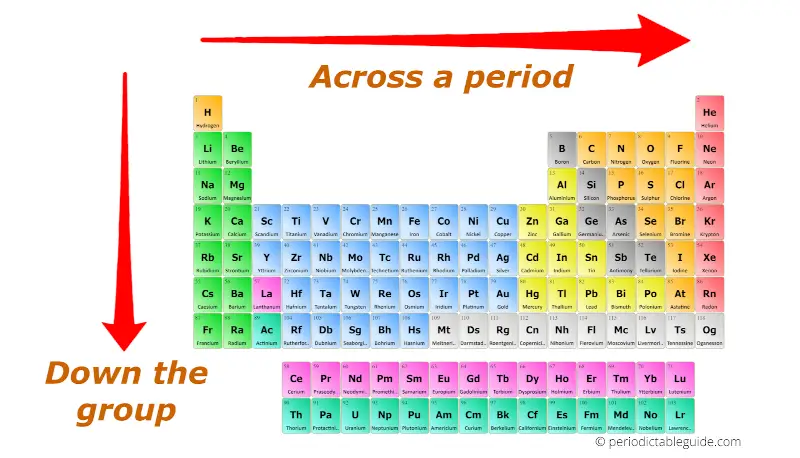
The periodic table is arranged in rows (periods) and columns (groups). Each element occupies a specific position based on its atomic number, representing the number of protons in its nucleus. Periodic trends are the predictable patterns observed in the properties of elements as you move across a period or down a group.
Key Periodic Trends:
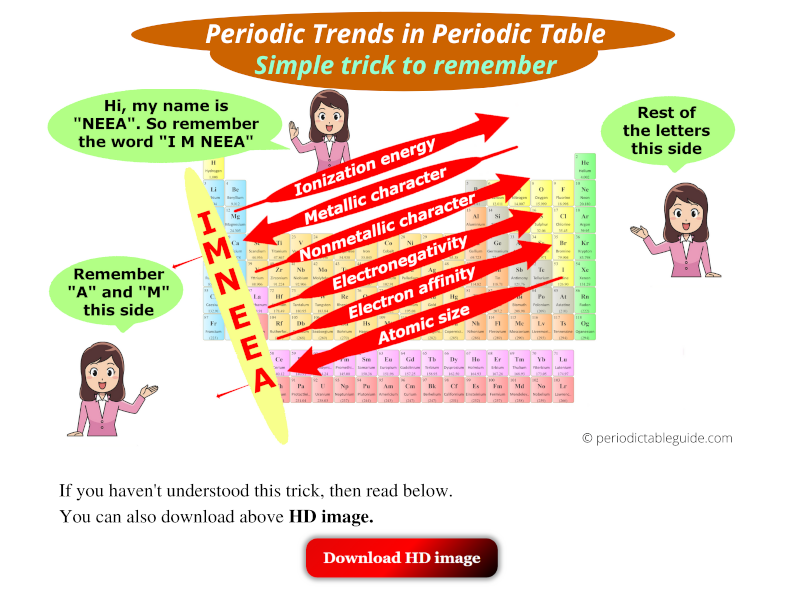
- Ionization Energy: The energy required to remove an electron from a gaseous atom in its ground state. Ionization energy generally increases across a period (due to increasing nuclear charge and decreasing atomic radius) and decreases down a group (due to increasing atomic radius and shielding effect).
- Electron Affinity: The change in energy when an electron is added to a neutral atom to form a negative ion. Electron affinity generally increases across a period (due to increasing nuclear charge and decreasing atomic radius) and decreases down a group (due to increasing atomic radius and shielding effect).
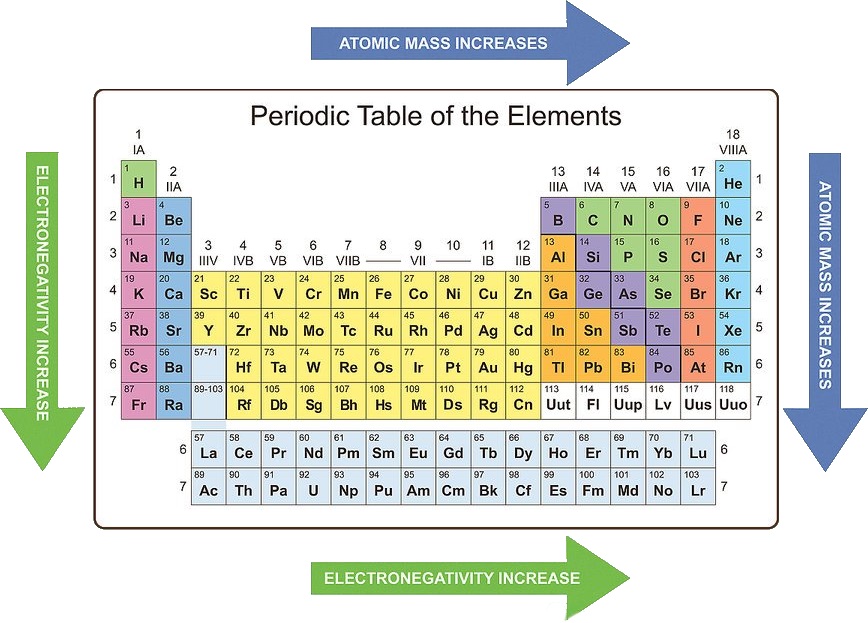
- Electronegativity: The ability of an atom to attract electrons in a chemical bond. Electronegativity generally increases across a period (due to increasing nuclear charge and decreasing atomic radius) and decreases down a group (due to increasing atomic radius and shielding effect).
- Atomic Radius: The distance between the nucleus of an atom and its outermost electron shell. Atomic radius generally decreases across a period (due to increasing nuclear charge pulling electrons closer) and increases down a group (due to the addition of electron shells).
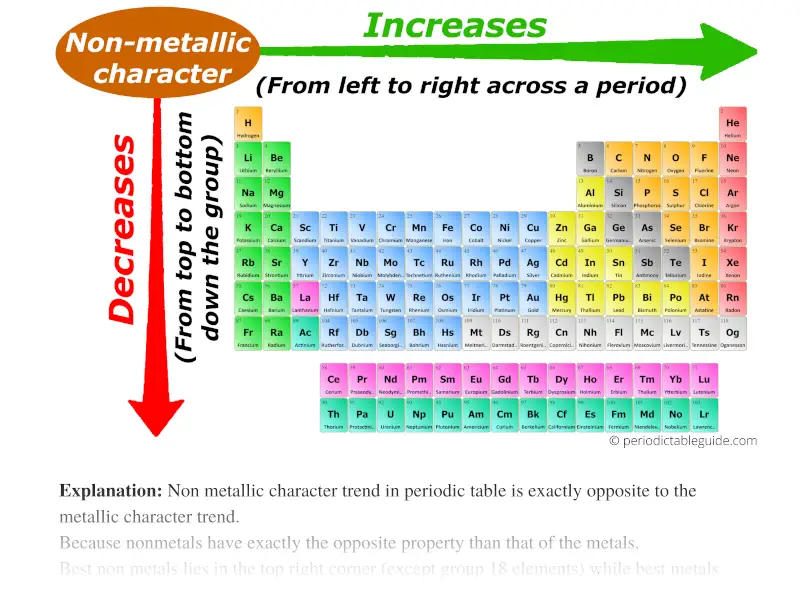
- Metallic Character: The tendency of an element to lose electrons and form positive ions. Metallic character generally decreases across a period (due to increasing electronegativity) and increases down a group (due to decreasing ionization energy).
Utilizing Periodic Trends Gizmo
While a specific answer key for a 2025 version of the Gizmo might not be readily available, the core principles of periodic trends remain consistent. The Gizmo likely allows users to manipulate various parameters, such as atomic number, electron configuration, and visualize the resulting changes in properties.
Key Benefits of Using Periodic Trends Gizmo:
- Interactive Learning: The Gizmo provides a dynamic and engaging way to learn about periodic trends. Users can manipulate variables and observe the resulting changes in properties.
- Visual Representation: The Gizmo likely utilizes visual representations to illustrate the trends, making them easier to understand and remember.
- Practical Applications: The Gizmo can be used to solve problems and predict the behavior of elements in various chemical reactions.
- Self-Paced Learning: Students can work at their own pace and revisit concepts as needed.
Related Searches
- Periodic Trends Gizmo Answer Key: This search likely leads to resources that provide answers to specific questions within the Gizmo or explanations of the concepts covered.
- Periodic Trends Worksheet Answers: This search may lead to worksheets with practice problems related to periodic trends, along with their solutions.
- Periodic Trends Quiz: This search can direct you to quizzes or assessments designed to test your understanding of periodic trends.
- Periodic Trends Chart: This search may lead to various charts and diagrams illustrating periodic trends, including their trends across periods and groups.
- Periodic Trends Explained: This search will lead to articles, videos, and other resources providing explanations of periodic trends and their underlying causes.
- Periodic Trends Examples: This search may lead to examples of how periodic trends manifest in real-world applications, such as in the reactivity of metals or the formation of ionic compounds.
- Periodic Trends and Chemical Bonding: This search will explore the connection between periodic trends and the types of chemical bonds formed between elements.
- Periodic Trends and Properties of Elements: This search will delve into how periodic trends influence the physical and chemical properties of elements.
FAQs
Q: Why are periodic trends important in chemistry?
A: Periodic trends provide a framework for understanding the chemical behavior of elements. They help predict how elements will react with each other and form compounds, making it possible to design and synthesize new materials.
Q: How can I use the Periodic Trends Gizmo effectively?
A: Experiment with different elements and observe how their properties change across periods and groups. Focus on understanding the underlying reasons for these trends, such as the increasing nuclear charge and shielding effect.
Q: What are some common misconceptions about periodic trends?
A: One common misconception is that all trends increase or decrease uniformly across a period or group. However, there can be exceptions and irregularities due to factors like electron configuration and orbital filling.
Q: How can I use periodic trends to predict the properties of an element?
A: By knowing the position of an element on the periodic table, you can predict its ionization energy, electronegativity, atomic radius, and other properties. You can also use these trends to predict the type of chemical bonds it will form.
Tips
- Visualize the Trends: Utilize diagrams and charts to visualize the trends across periods and groups. This helps to solidify your understanding.
- Practice with Examples: Work through examples of how periodic trends influence the properties of elements and their reactions.
- Connect Trends to Atomic Structure: Relate the trends to the underlying atomic structure, such as electron configuration and nuclear charge.
Conclusion
Understanding periodic trends is crucial for comprehending the behavior of elements and predicting their interactions. The Periodic Trends Gizmo provides a valuable tool for exploring these trends interactively. By utilizing this resource and applying the concepts learned, students and educators can gain a deeper understanding of this fundamental aspect of chemistry.
.PNG)
/periodictrendstable-5c4a46614cedfd000187c5db.jpg)


Closure
Thus, we hope this article has provided valuable insights into Understanding Periodic Trends: A Comprehensive Guide. We thank you for taking the time to read this article. See you in our next article!